
This element was discovered by J. Berzelius and W. Hisinger in Sweden, in 1803. It was, however, isolated only much later (1875). The name comes from the goddess Ceres, whose name was givento an asteroid discoveredin 1801.
Ionization energies
CeI 5.5 eV, CeII 10.8 eV, CeIII 20.2 eV, Ce IV 36.8 eV.
Absorption lines of CeI
The strongest line of Ce I (5699) is not present in the solar spectrum.
Absorption lines of CeII
| 4628(1) | 5274(15) | 5610(26) | ||||
| Group | V | V | III | III | Ib | |
| F 0 | 0.024(Ib) | |||||
| F 5 | 0.014 | |||||
| G 0 | 0.02 | 0.046 | ||||
| G 2 | 0.008 | 0.032 | ||||
| S | 0.014 | 0.006 | blend | |||
| G 5 | 0.038 | |||||
| G 8 | 0.074 | |||||
| K 0 | 0.062 | 0.018 | ||||
| K 2 | 0.028 | 0.095 | ||||
| K 3 | 0.063 | 0.098 | ||||
| K 5 | 0.123 | |||||
| M 2 | 0.198 | |||||
| Group | III | Ib |
| G 1 | 0.031 | |
| G 2 | 0.027,0.035 | |
| S | 0.002 | |
| G 5 | 0.033 | |
| G 8 | 0.042 | |
| K 0 | 0.004 | |
| K 2 | 0.047 | |
| K 5 | 0.010 | 0.034,0.068 |
| M 0 | 0.013 | |
CeII lines (see for instance the line at 5610) are present in stars later than G-type. A positive luminosity effect is observed.
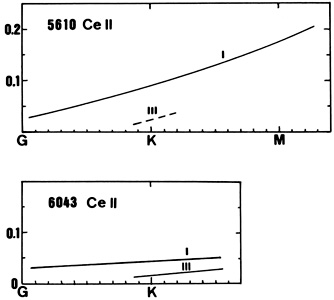 |
Behavior in non-normal stars
CeII is strong in Ap stars of the Cr-Eu-Sr subgroup
(Adelman 1973b)
(W(4628)  0.030 -
0.100). Of all rare earths, this is the most
abundant one in all Ap stars
(Cowley 1976).
Ce III also has been observed in some of these stars
(Aikman et al. 1979,
Cowley 1984).
0.030 -
0.100). Of all rare earths, this is the most
abundant one in all Ap stars
(Cowley 1976).
Ce III also has been observed in some of these stars
(Aikman et al. 1979,
Cowley 1984).
CeII is also strong in Am stars (Smith 1973, 1974), where the W values are much larger than in normal stars of the same temperature. As an example W(4137) is about 0.100 in late Am stars, whereas for normal F 0 dwarfs, W(4137) is only 0.080.
As with other rare earths, CeII lines are intensified in Ba stars, which leads to large overabundances (Lambert 1985). A typical value is W(4628) = 0.16 for a K O III Ba star (Danziger 1965).
CeII is also enhanced in at least one early C star (Dominy 1984).
CeII has been observed in G- and K-type metal-weak stars by Gilroy et al. (1988). It is overabundant with respect to iron (see also Part Two, section 2.2).
Isotopes
Ce has 19 isotopes and isomers, four of them stable, namely Ce 136, 138, 140 and 142. In the solar system most of the Ce (88%) is in the form of Ce140.
Origin
Ce136 and Ce138 are produced by the p process, Ce142 by the r process and Ce140 by either the r or the s process.
Published in "The Behavior of Chemical Elements in Stars", Carlos Jaschek and Mercedes Jaschek, 1995, Cambridge University Press.