
The element was discovered by Vauquelin in Paris in 1797. The name cones from the Greek chromos (color), because of the colored salts it forms.
Ionization energies
CrI 6.8 eV, CrII 16.5 eV, CrIII 31.0, Cr IV 49.1 eV, CrV 69.3 eV, CrVI 90.6
Cr is well represented in stellar spectra, especially those of later types. In the solar spectrum, Cr I lines are more numerous than those of any other newutral element, except Fe.
Absorption lines of CrI
| 4254(1) | 5345(18) | |||
| Group | V | Ib | III | Ib |
| B 9 | 0.03 | |||
| A 0 | 0.02 | |||
| A 2 | 0.05 | |||
| A 7 | 0.18 | |||
| F 0 | 0.19 | 0.22(Ia) | ||
| F 2 | 0.26 | |||
| F 4 | 0.19 | |||
| F 5 | 0.19 | 0.35 | ||
| F 6 | 0.24 | |||
| F 8 | 0.24 | 0.43 | ||
| G 0 | 0.27 | 0.26 | ||
| G 1 | 0.37 | 0.29 | ||
| G 2 | 0.30 | 0.29 | ||
| S | 0.393 | 0.107 | ||
| G 5 | 0.45 | 0.30 | ||
| G 8 | 0.29 | |||
| K 0 | 1.27 | 0.14 | ||
| K 2 | 0.40 | 0.46 | ||
| K 3 | 0.33 | |||
| K 5 | 2.60 | 0.62 | ||
| M 0 | 0.65 | |||
| M 2 | 0.59 | |||
| M 2.5 | 0.38 | |||
| M 3 | 0.54 | |||
| M 4 | 0.47,0.84 | |||
| M 5 | 0.72 | |||
| M 7 | (1.16) | |||
| Group | V | III | Ib |
| F 4 | 0.12 | ||
| F 5 | 0.10 | 0.09 | |
| F 6 | 0.12 | ||
| F 8 | 0.15 | 0.23 | |
| G 0 | 0.14 | ||
| G 1 | 0.21 | ||
| G 2 | 0.15 | ||
| S | 0.154 | ||
| G 5 | 0.21 | ||
| G 8 | 0.20(IV) | 0.26 | |
| K 0 | 0.28 | 0.21 | |
| K 2 | 0.38 | ||
| M 0 | 0.49 | ||
| M 2.5 | 0.36 | ||
CrI (for instance the lines at 4254 and 5409) appears in A-type spectra and grows monotonically toward later types. A small positive luminosity effect seems to exist for the line at 4254. According to Keenan and McNeil (1976) the luminosity effect becomes negative after K.
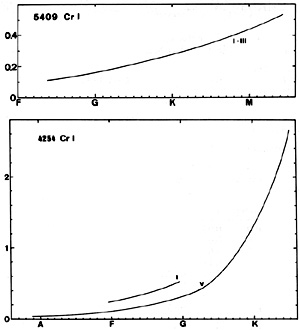 |
The most intense infrared line of CrI is that at 9290(29).In the sun, W = 0.065.
Absorption lines of CrII
| 455(44) | 5502(50) | ||||
| Group | V | Ib | V | III | Ib |
| B 5 | 0.005 | ||||
| B 7 | 0.023 | ||||
| B 8 | 0.036 | ||||
| B 9 | 0.080 | ||||
| B 9.5 | 0.047,064 | ||||
| A 0 | 0.076 | 0.19,0.15(Ia) | |||
| A 1 | 0.097 | ||||
| A 2 | 0.15,0.10 | 0.658(0) | |||
| A 3 | 0.340(0) | ||||
| A 7 | 0.17 | ||||
| F 0 | 0.15 | 0.62(Ia) | |||
| F 2 | 0.26 | ||||
| F 4 | 0.13 | ||||
| F 5 | 0.14 | 0.36 | |||
| F 6 | 0.11 | ||||
| F 8 | 0.11 | 0.46 | |||
| G 0 | 0.09 | 0.00 | 0.00 | 0.072 | |
| C 1 | 0.14 | ||||
| G 2 | 0.07 | 0.017 | 0.03 | ||
| S | 0.066 | 0.023 | |||
| G 5 | 0.062 | ||||
| G 8 | 0.095 | 0.058 | |||
| K 0 | 0.055 | 0.017 | 0.035 | ||
| K 2 | 0.027 | 0.078 | |||
| K 3 | 0.049 | 0.095 | |||
| K 5 | 0.06 | 0.129 | |||
| Group | V | Ib |
| S | 0.049 | |
| G 5 | 0.144 | |
| G 8 | 0.161 | |
| K 2 | 0.181 | |
| K 5 | 0.153,0.197 | |
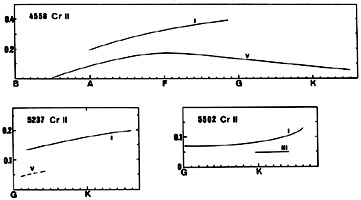
CrII appears in late B-type dwarfs, increases to a flat maximum in early F-type and declines toward late dwarfs (see for instance the line at 4558). In supergiants the maximum is displaced toward later types. A positive luminosity effect exists.
 |
Emission lines of CrII
Both permitted and forbidden Cr emission lines are observed in B[e] stars (see Swims (1973) for the analysis of a typical object). They are also observed in the ultraviolet region of T Tau stars (Appenzeller et al. 1980) and in symbiotic stars (Swings and Struve 1941a).
Absorption lines of CrIV and CrV
CrIV and CrV have been identified in the ultraviolet spectra of O3-type stars (Dean and Bruhweiler 1985).
Behavior in non-normal stars
CrII is strong in Ap stars of the Cr-Fu-Sr subgroup. Typically W(4558) = 0.30. It should, however, be observed that the strongest CrII laboratory lines are not the CrII lines that are most enhanced in these stars (Jaschek and Jaschek 1974).
Cr behaves in a manner parallel to that of Fe in metal-weak dwarfs (Magain 1989) and in globular cluster stars (Wheeler et al. 1989).
Cr can be considered to be a typical `metal'.
Isotopes
Cr has four stable isotopes and five short-lived ones. The stable ones are Cr 50, 52, 53 and 54. In the solar system they are present with 4%, 84%, 9% and 3% abundances respectively.
Origin
Cr 50, 52 and 53 are produced by explosive nucleosynthesis, whereas Cr54 is produced by nuclear statistical equilibrium.
Published in "The Behavior of Chemical Elements in Stars", Carlos Jaschek and Mercedes Jaschek, 1995, Cambridge University Press.