


2.2. Connection with thermal instability
There is an intimate connection between the existence of thermal phases and the thermal stability of a system: any system exhibiting multiphase equilibria must be thermally unstable over a range of thermodynamic parameters. The thermal stability of astrophysical gases was first studied systematically by Field (1965). His instability criterion was generalized to non-equilibrium systems by Balbus (1986a), who found the following condition for instability:
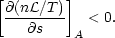
| (2) |
Here s is the entropy per hydrogen nucleus and A is some
thermodynamic variable which
is held constant during the perturbation. In equilibrium,
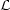 = 0 and this reduces
to Field's instability criterion
= 0 and this reduces
to Field's instability criterion
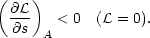
| (3) |
In general, s is a complicated function of n, T,
and the state of ionization of the
gas. However, in many applications the gas is almost completely ionized
and the entropy
function may be approximated by the expression for an ideal gas,
s ~ lnpV5/3 + const. If
A is some power law combination of p and V, then
T( s /
s /
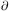 T)A
is a constant specific heat which is positive for cases of interest. The
instability criterion then becomes
T)A
is a constant specific heat which is positive for cases of interest. The
instability criterion then becomes
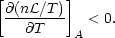
| (4) |
Since the cooling time is proportional to
T / n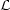 , this
criterion can be rephrased as stating
that instability occurs if the cooling time increases with temperature
(Balbus 1986a).
, this
criterion can be rephrased as stating
that instability occurs if the cooling time increases with temperature
(Balbus 1986a).
If the gas is in equilibrium
(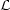 = 0), the instability
criterion (4) reduces to
= 0), the instability
criterion (4) reduces to
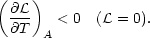
| (5) |
Field (1965)
showed that for the equilibrium case the isobaric criterion
(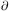
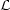 /
/
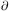 T)p
< 0 is
usually the correct one to apply. However, if the system is large enough
that the sound
crossing time is long compared to the heating or cooling times, then for
long wavelengths the isochoric criterion
(
T)p
< 0 is
usually the correct one to apply. However, if the system is large enough
that the sound
crossing time is long compared to the heating or cooling times, then for
long wavelengths the isochoric criterion
(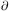
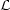 /
/
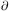 T)V
< 0 is applicable.
T)V
< 0 is applicable.
The stability criterion (5) may be interpreted geometrically in terms of
the equilibrium
curve (Figure 1). Typically the
cooling region
(n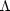 >
>
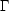 )
lies above the heating region
because the cooling rate usually increases faster with n and
T than does the heating rate.
If, on the other hand, the heating region lay above the cooling region,
then over much of the
curve (wherever p(V) is single-valued) one would have
(
)
lies above the heating region
because the cooling rate usually increases faster with n and
T than does the heating rate.
If, on the other hand, the heating region lay above the cooling region,
then over much of the
curve (wherever p(V) is single-valued) one would have
(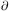
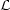 /
/
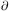 T)V < 0 and the equilibrium
would be isochorically unstable. In this case systems large enough that
the sound crossing
time is much greater than the heating and cooling times could be
unstable even where
smaller systems are isobarically stable. This situation does not arise
in practice and we
therefore assume that the cooling region lies above the heating region
in the p - V plane, as shown in
Figure 1.
T)V < 0 and the equilibrium
would be isochorically unstable. In this case systems large enough that
the sound crossing
time is much greater than the heating and cooling times could be
unstable even where
smaller systems are isobarically stable. This situation does not arise
in practice and we
therefore assume that the cooling region lies above the heating region
in the p - V plane, as shown in
Figure 1.
The slope of the equilibrium curve in the p - V plane is directly related to the stability of the system since
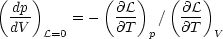
| (6) |
(Field 1965). For cases in which p(V) is single valued (as in Fig. 1 a-c), the condition that the cooling region lie above the heating region implies that the system is isochorically stable, so that the denominator in equation (6) is positive; hence, in this case isobarically stable regions have a negative slope in the p - V plane, whereas unstable regions have a positive slope. The condition for a multiphase equilibrium is that V(p) be a multivalued function, which is equivalent to having d lnp / d ln V change sign. Thus, a necessary and sufficient condition for the existence of a multiphase equilibrium is that the system be thermally unstable over a finite range of V. This proves the assertion at the beginning of this section. Fig. 1 d illustrates a case in which p(V) is multivalued over a range in V. Such a system can exhibit both isochoric and isobaric instability, where the equilibrium curve has a negative slope in the p - V plane.
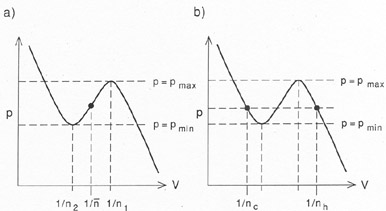
A system with two stable phases (e.g.,
Fig. 1b) may be used to
illustrate the inevitability
of multiple phases under certain circumstances. A characteristic feature
of two-phase
systems is that the cold phase cannot exist below some minimum pressure
pmin, while the
hot phase cannot exist above some maximum pressure
pmax. The condition that there be
two stable phases implies that
pmax > pmin. Now consider
a homogeneous system with a density
n1 <  < n2, as shown in
Fig. 2a. Such a system is clearly
unstable in its
homogeneous state. However, it is always possible to stabilize the
system by making it
inhomogeneous, while keeping the mean density constant
(Fig. 2b). The trick is to put
most of the mass in the cold phase, with density
nc > n2,
while a small fraction of the
matter forms a hot intercloud medium, with density
nh < n1 and
temperature Th. Pressure balance requires
nc / nh = Th /
Tcl. If f is the filling factor in cold gas,
then the mean density constraint is
< n2, as shown in
Fig. 2a. Such a system is clearly
unstable in its
homogeneous state. However, it is always possible to stabilize the
system by making it
inhomogeneous, while keeping the mean density constant
(Fig. 2b). The trick is to put
most of the mass in the cold phase, with density
nc > n2,
while a small fraction of the
matter forms a hot intercloud medium, with density
nh < n1 and
temperature Th. Pressure balance requires
nc / nh = Th /
Tcl. If f is the filling factor in cold gas,
then the mean density constraint is
 = (1 -
f)nh + fnc, and
f satisfies
Tcl / Th << f <<
1 if n1 <<
= (1 -
f)nh + fnc, and
f satisfies
Tcl / Th << f <<
1 if n1 <<
 <<
n2.
<<
n2.
|
Figure 2. Inevitability of thermal
instability in a system with a fixed
mean density |