


2.11. Electrostatic Energy
The effect of the electrostatic interaction on the binding energies of atomic nuclei relative to free protons and electrons is taken into account in category 6, and the electrostatic contribution to the binding energies of white dwarfs is (in principle) part of category 5. The molecular binding energy in objects ranging from dust to asteroids that are held together by the electrostatic interaction deserves separate mention.
The molecular binding energy relative to free atoms in condensed matter is roughly 1 eV per atom. The product of this mass fraction, ~ 10-10, with entry 3.12 is
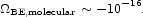 |
(129) |
This is the binding energy of condensed matter physics outside strongly self-gravitating systems. We refrain from entering it in Table 1 because the estimate is so small and uncertain, but we offer for comparison the binding energy of the electrons in atoms.
The electrostatic binding energy of the electrons in an O VI atom is 1.6 keV, and the addition of the other five electrons in a neutral oxygen atom increases the binding energy by only 27 percent. That is, it is a reasonable approximation to ignore the states of ionization of the heavy elements and the electrostatic binding energy of neutral hydrogen and helium. The sum over the heavy element masses in entries 1, 2, 4, 5, and 6 in Table 3, weighted by the neutral atomic binding energies of 15 cosmically conspicuous elements, is
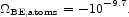 |
(130) |
This is some six orders of magnitude larger than the molecular binding energy in equation (129), and comparable in value to the smallest entries in Table 1.