| (1)
| An atomic nucleus consists of Z protons and N
neutrons, where Z is the atomic
number defining the charge of the nucleus, the number of
electrons in the neutral
atom and hence the chemical element, and Z + N = A,
the mass number of the
nuclear species. Protons and neutrons are referred to
collectively as nucleons.
Different values of A or N for a given element lead to different
isotopes, while
nuclei with the same A and different Z are referred to as
isobars. A given nuclear
species is usually symbolised by the chemical symbol with Z as an
(optional) lower and A as an upper prefix,
e.g. 5626Fe.
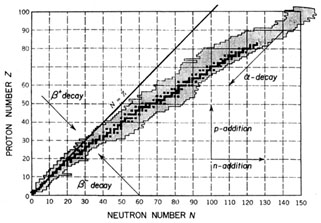
|
Fig. 1.2. Chart of the nuclides, in which Z
is plotted against N. Stable nuclei are shown in
dark shading and known radioactive nuclei in light shading.
Arrows indicate directions of some
simple nuclear transformations. After K.S. Krane, Introductory
Nuclear Physics, ©1988 by John
Wiley & Sons. Reproduced by permission of John Wiley & Sons, Inc.
|
Stable nuclei occupy a `` -stability valley'' in the Z, N plane
(see Fig. 1.2),
where one can imagine energy (or mass) being plotted along a
third axis perpendicular to the paper. Various processes, some of which are
shown in the figure, transform one nucleus into another. Thus, under normal
conditions, a
nucleus outside the valley undergoes spontaneous decays, while in
accelerators,
stars and the early universe nuclei are transformed into one
another by various reactions. -stability valley'' in the Z, N plane
(see Fig. 1.2),
where one can imagine energy (or mass) being plotted along a
third axis perpendicular to the paper. Various processes, some of which are
shown in the figure, transform one nucleus into another. Thus, under normal
conditions, a
nucleus outside the valley undergoes spontaneous decays, while in
accelerators,
stars and the early universe nuclei are transformed into one
another by various reactions.
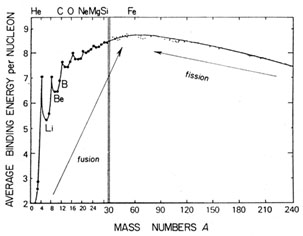
|
Fig. 1.3. Binding energy per nucleon as a
function of mass number. Adapted from Rolfs & Rodney (1988).
|
|
| (2)
| The binding energy per nucleon varies with A along the
stability valley as shown
in Fig. 1.3, and this has the following
consequences:
(a) Since the maximum binding energy per nucleon is possessed by
62Ni,
followed closely by 56Fe, energy is released by either fission of
heavier or fusion
of lighter nuclei. The latter process is the main source of
stellar energy, with
the biggest contribution (7 MeV per nucleon) coming from the
conversion of hydrogen into helium (H-burning).
(b) Some nuclei are more stable than others, e.g. the  -particle
nuclei 4He,
12C, 16O, 20Ne, 24Mg,
28Si, 32S, 36Ar,
40Ca. Nuclei with a couple of A-values
(5 and 8) are violently unstable, owing to the nearby helium
peak. Others are stable but only just: examples are D, 6,7Li,
9Be and 10,11B, which are destroyed
by thermonuclear reactions at relatively low temperatures. -particle
nuclei 4He,
12C, 16O, 20Ne, 24Mg,
28Si, 32S, 36Ar,
40Ca. Nuclei with a couple of A-values
(5 and 8) are violently unstable, owing to the nearby helium
peak. Others are stable but only just: examples are D, 6,7Li,
9Be and 10,11B, which are destroyed
by thermonuclear reactions at relatively low temperatures.
| (3)
| Nuclear reactions involving charged particles
(p,  etc.)
require them to have
enough kinetic energy to get through in spite of the
electrostatic repulsion of the
target nucleus (the ``Coulomb barrier''); the greater the charges,
the greater the
energy required. In the laboratory, the energy is supplied by
accelerators, and
analogous processes are believed to occur in reactions induced in
the ISM by
cosmic rays (see Chapter 9). In the interiors of stars, the
kinetic energy exists by
virtue of high temperatures (leading to thermonuclear reactions)
and when one
fuel (e.g. hydrogen) runs out, the star contracts and becomes
hotter, eventually
allowing a more highly charged fuel such as helium to ``burn''. etc.)
require them to have
enough kinetic energy to get through in spite of the
electrostatic repulsion of the
target nucleus (the ``Coulomb barrier''); the greater the charges,
the greater the
energy required. In the laboratory, the energy is supplied by
accelerators, and
analogous processes are believed to occur in reactions induced in
the ISM by
cosmic rays (see Chapter 9). In the interiors of stars, the
kinetic energy exists by
virtue of high temperatures (leading to thermonuclear reactions)
and when one
fuel (e.g. hydrogen) runs out, the star contracts and becomes
hotter, eventually
allowing a more highly charged fuel such as helium to ``burn''.
There is no Coulomb barrier for neutrons, but free neutrons are
unstable so
that they have to be generated in situ, which again demands high
temperatures.
| |

