


Above, we made a distinction between particles and fields. Electrons, protons, and many other particles carry an electric charge that creates a surrounding electromagnetic field. Charged particles oscillating in an atom or an antenna create light or radio waves, which propagate away, carrying energy and information. With the invention of quantum theory and the discovery of new particles and fields, the distinction between particle and field has become somewhat blurred.
We know that the free field consists of photons: a wave with definite
frequency can give or take energy away in discrete bundles - quanta
(as they were initially called), i.e., photons with energy E =
h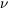 and the
corresponding momentum. They are particle-like. The new W and Z
fields, like the other hypothetical vector fields, behave experimentally
like particles.
and the
corresponding momentum. They are particle-like. The new W and Z
fields, like the other hypothetical vector fields, behave experimentally
like particles.
At the beginning of this century, "true particles" like electrons and protons were assumed to exist forever. The electron emitted from the cathode of a cathode-ray tube already existed in the metallic cathode. The photon emitted by an antenna did not exist, but was created when the radio waves were emitted. This was thought to be the most important distinction between particles and fields.
However, this distinction became blurred in the thirties: we know that individual electrons are created in beta decay and that positron-electron pairs can be created from vacuum if there is enough energy. Electric charge is conserved, but individual particles are not.
There is still one distinction that has not yet become blurred.
Electrons, positrons, neutrinos, protons, and neutrons all have spin
(intrinsic angular momentum) n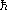 / 2 (i.e., half-integer multiples of
/ 2 (i.e., half-integer multiples of 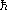 =
h / 2
=
h / 2 = 1.05 x
10-27 g cm2 s-1,
the Planck constant in the notation
now in common use). It has been both theoretically and experimentally
established that particles with half-integer spin obey the Pauli exclusion
principle. Two or more particles cannot have an identical set of
quantum numbers.
= 1.05 x
10-27 g cm2 s-1,
the Planck constant in the notation
now in common use). It has been both theoretically and experimentally
established that particles with half-integer spin obey the Pauli exclusion
principle. Two or more particles cannot have an identical set of
quantum numbers.
Particles corresponding to a vector field have spin
1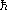 , while gravitons
- the quanta for the free gravitational field - have spin
2
, while gravitons
- the quanta for the free gravitational field - have spin
2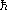 . The scalar
fields to be discussed below have spin zero.
. The scalar
fields to be discussed below have spin zero.
The property these latter particles have in common is integral spin (in
units of 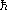 ). They are called
bosons and do not obey the Pauli principle.
In fact, the opposite is true: bosons like to be in the same state. This is
the basis of laser theory; suppose that an electromagnetic wave consisting
of N identical photons already exists and suppose that this wave hits
an excited atom. The probability that the atom will emit a photon with
the same characteristics as those in the wave (so that the wave consists
of N + 1 photons) is a factor of N + 1 greater than the
probability for emission in any other direction.
). They are called
bosons and do not obey the Pauli principle.
In fact, the opposite is true: bosons like to be in the same state. This is
the basis of laser theory; suppose that an electromagnetic wave consisting
of N identical photons already exists and suppose that this wave hits
an excited atom. The probability that the atom will emit a photon with
the same characteristics as those in the wave (so that the wave consists
of N + 1 photons) is a factor of N + 1 greater than the
probability for emission in any other direction.
Photons like to be together and, once they are together, the individual character of each photon is destroyed.
Individual hard gamma ray photons behave like particles, but the multiphoton radiation from an antenna behaves like a classical field. The moral to be drawn here is that classical field theory is still useful. Classical field theory can stand on its own, despite the existence of quantum theory. The classical theory can be thought of as the limiting case of quantum theory. This is true in the trivial case of the mechanics of a large body - a planet in the solar system or a shaft in a car.
This is also true for a field theory in which the field consists of many individual photons or quanta (of course, only for Bose fields, i.e., fields with integer spin). Classical field theory has proven useful in electrical engineering, which is extremely important in twentieth century technology (electromagnetic field, spin one), celestial mechanics (gravitation, spin two), and it is conjectured that it will also be useful for the scalar field (spin zero), whose importance to cosmology will be explained below.