


5.2.1. Ionization equilibrium
In equilibrium, the ionization state is determined by the balance between processes that produce or destroy each ion:
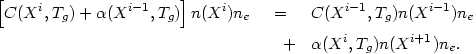
| (5.15) |
Here n(Xi) is the number density of the ion
Xi (x is the element), Tg is the
electron temperature, and c(Xi,
Tg) and
 (Xi,
Tg) are the rate coefficients
for collisional ionization out of ion Xi and
recombination into ion Xi, respectively.
(Xi,
Tg) are the rate coefficients
for collisional ionization out of ion Xi and
recombination into ion Xi, respectively.
The collisional ionization rate is the sum of two processes: direct collisional ionization and collisional excitation of inner shell electrons to autoionizing levels which decay to the continuum. This last process is often referred to as autoionization. Recombination is also the sum of two processes, radiative and dielectronic recombination. Recent compilations of ionization and recombination rates and discussions of their accuracy include Mewe and Gronenschild (1981), Shull and Van Steenberg (1982), and Hamilton et al. (1983).
The electron density dependence drops out of equation (5.15), and the equilibrium ionization state of a diffuse plasma depends only on the electron temperature. Tables of ionization fractions of various elements are given by Shull and Van Steenberg (1982). Generally, each ionization fraction reaches a maximum at a temperature that is some fraction of its ionization potential. At the temperatures which predominate in clusters, iron is mainly in the fully stripped, hydrogenic, or heliumlike stages.