


3.2. "Exact-Statistical" Approach
As discussed in Section 3.1, in most cases soon after the photoabsorption an isolated nanoparticle (or large molecule) converts almost all of the initial photoexcitation energy to vibrational energy of the highly vibrationally excited ground electronic state, and hence for both neutrals and ions, IR emission is always the dominant deactivation process. Therefore, it is reasonable to model the stochastic heating of a nanoparticle in terms of pure vibrational transitions.
Ideally, if both the vibrational energy levels and the level-to-level transition probabilities were known, we could (at least in principle) solve for the statistical steady-state populations of the different energy levels of grains illuminated by a known radiation field. However, this level of detailed information is generally unavailable, for even the smallest and simplest PAH molecules.
Draine & Li (2001)
developed an "exact-statistical" theory
for modeling the photoexcitation and emission processes of
nanoparticles. In this theory, the state of the grain is
characterized by its vibrational energy E.
Since there are too many vibrational energy levels
to consider individually, they are grouped into
(M + 1) "bins" j = 0,..., M,
where the j-th bin is
[Ej, min, Ej, max),
with representative energy
Ej  (Ej, min + Ej, max) / 2, and width
(Ej, min + Ej, max) / 2, and width
 Ej
Ej
 (Ej,
max - Ej, min) (see
Fig. 3 for illustration).
Let Pj be the probability of finding the grain in bin
j.
The probability vector Pj evolves according to
dPi / dt =
(Ej,
max - Ej, min) (see
Fig. 3 for illustration).
Let Pj be the probability of finding the grain in bin
j.
The probability vector Pj evolves according to
dPi / dt =
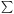 j
j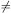 i
Tij Pj -
i
Tij Pj -
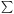 j
j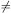 i Tji
Pi for i = 0, 1,..., M,
where the transition matrix element Tij is
the probability per unit time for
a grain in bin j to make a transition to one of the levels
in bin i. If we define the diagonal elements of T to be
Tii
i Tji
Pi for i = 0, 1,..., M,
where the transition matrix element Tij is
the probability per unit time for
a grain in bin j to make a transition to one of the levels
in bin i. If we define the diagonal elements of T to be
Tii
 -
-
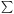 j
j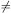 i Tji,
then under the steady state condition
(i.e. dPi / dt = 0 for i = 0, 1,...,
M) the state probability evolution equation becomes
i Tji,
then under the steady state condition
(i.e. dPi / dt = 0 for i = 0, 1,...,
M) the state probability evolution equation becomes
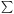 j=0M
Tij Pj
= 0 for i = 0,..., M.
Combining this with the normalization condition
j=0M
Tij Pj
= 0 for i = 0,..., M.
Combining this with the normalization condition
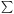 j=0M
Pj = 1, we obtain a set of M linear equations
for the first M elements of Pj:
j=0M
Pj = 1, we obtain a set of M linear equations
for the first M elements of Pj:
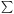 j=0M-1
(Tij - TiM)Pj = -
TiM
for i = 0,..., M - 1, which we solve using the bi-conjugate
gradient (BiCG) method. The remaining undetermined element
PM is obtained by
PM = - (TMM)-1
j=0M-1
(Tij - TiM)Pj = -
TiM
for i = 0,..., M - 1, which we solve using the bi-conjugate
gradient (BiCG) method. The remaining undetermined element
PM is obtained by
PM = - (TMM)-1
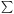 j=0M-1
TMj Pj.
j=0M-1
TMj Pj.
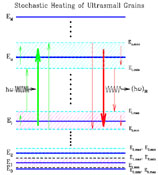 |
Figure 3. Schematic diagram of the
vibrational excitation and relaxation processes in nanoparticles (or large
molecules). The vibrational energy levels are
grouped into (M + 1) "bins" j = 0,..., M,
where the j-th bin is [Ej, min,
Ej, max), with representative energy
Ej |
For a given starlight energy density uE,
the state-to-state transition rates
Tji for transitions i
 j can
be determined from photon absorptions and photon emissions.
The rate for upward transitions l
j can
be determined from photon absorptions and photon emissions.
The rate for upward transitions l
 u
is just the absorption rate of photons with such an energy
that they excite the grain from bin l just to bin u.
If the bin width is sufficiently small
(i.e., if max[
u
is just the absorption rate of photons with such an energy
that they excite the grain from bin l just to bin u.
If the bin width is sufficiently small
(i.e., if max[ El,
El,
 Eu]
<< [Eu - El]),
the l
Eu]
<< [Eu - El]),
the l
 u
excitation rate is simply
Tul
u
excitation rate is simply
Tul  Cabs(E) c uE
Cabs(E) c uE
 Eu
/ (Eu - El)
for u < M, where
Cabs(E) is the grain absorption
cross section at wavelength
Eu
/ (Eu - El)
for u < M, where
Cabs(E) is the grain absorption
cross section at wavelength
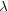 = hc / E
(h is the Planck function and c is the speed of light); and
TMl
= hc / E
(h is the Planck function and c is the speed of light); and
TMl  Cabs(E) c uE
Cabs(E) c uE
 EM
/ (EM - El) +
EM
/ (EM - El) +
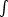 EM-E1
EM-E1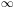 dE
Cabs(E) c uE /
(EM - El),
where the integral takes energy absorbed in transitions to levels
beyond the highest bin and allocates it to the highest bin (M).
For the special case of transitions u - 1
dE
Cabs(E) c uE /
(EM - El),
where the integral takes energy absorbed in transitions to levels
beyond the highest bin and allocates it to the highest bin (M).
For the special case of transitions u - 1
 u
we include "intrabin" absorptions:
u
we include "intrabin" absorptions:
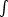 0
0 Eu-1 dE (1 -
E /
Eu-1 dE (1 -
E /  Eu-1)Cabs(E) c
uE / (Eu - Eu-1).
Correction for finite bin width, which is important when
the treatment is applied to grains with radii a
Eu-1)Cabs(E) c
uE / (Eu - Eu-1).
Correction for finite bin width, which is important when
the treatment is applied to grains with radii a
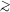 50 Å,
has been made by
Draine & Li (2001)
by introducing a Gul(E) factor (see Eqs.[15-25] of
Draine & Li 2001).
50 Å,
has been made by
Draine & Li (2001)
by introducing a Gul(E) factor (see Eqs.[15-25] of
Draine & Li 2001).
The rates for downward transitions u
 l
can be determined from a detailed balance analysis
of the Einstein A coefficient.
Similarly, if the bin width is sufficiently small, the u
l
can be determined from a detailed balance analysis
of the Einstein A coefficient.
Similarly, if the bin width is sufficiently small, the u
 l
de-excitation rate can be approximated as Tlu
l
de-excitation rate can be approximated as Tlu
 (8
(8 / h3
c2)
gl / gu
/ h3
c2)
gl / gu
 Eu
/ (Eu - El)
E3 ×
Cabs(E)[1 +
(h3 c2 /
8
Eu
/ (Eu - El)
E3 ×
Cabs(E)[1 +
(h3 c2 /
8 E3)
uE]
for l < u -1, where the uE-containing
term is the contribution
of stimulated emission, and the degeneracies gu and
gl
are the numbers of energy states in bins u and l,
respectively: gj
E3)
uE]
for l < u -1, where the uE-containing
term is the contribution
of stimulated emission, and the degeneracies gu and
gl
are the numbers of energy states in bins u and l,
respectively: gj
 N(Ej, max) - N(Ej,
min)
N(Ej, max) - N(Ej,
min)
 (dN / dE)Ej
(dN / dE)Ej
 Ej,
where (dN / dE)Ej
is the vibrational density of states at internal energy
Ej,
which corresponds to the number of ways of distributing this energy
between different modes of this grain. Again, we refer the reader to
Draine & Li (2001)
for finite bin width corrections as well as
"intrabin" radiation consideration (see Eqs.[29-31] of
Draine & Li 2001).
Ej,
where (dN / dE)Ej
is the vibrational density of states at internal energy
Ej,
which corresponds to the number of ways of distributing this energy
between different modes of this grain. Again, we refer the reader to
Draine & Li (2001)
for finite bin width corrections as well as
"intrabin" radiation consideration (see Eqs.[29-31] of
Draine & Li 2001).
It is seen from the above discussions that we require only Cabs(E), the degeneracies gj, and the starlight spectrum uE to completely determine the transition matrix Tij. A molecule containing Na atoms will have Nm = 3Na - 6 distinct vibrational modes (plus 3 translational degrees of freedom and 3 rotational degrees of freedom). If the molecule is approximated as a set of Nm harmonic oscillators, and the frequencies of all normal modes of this molecule are known, we can calculate N(E), the number of distinct vibrational states with total vibrational energy less than or equal to E, using the Beyer-Swinehart algorithm (Beyer & Swinehart 1973; Stein & Rabinovitch 1973). So far, the frequencies of these normal modes have been computed only for a small number of PAHs, with some frequencies determined experimentally, but mode spectra are not yet available for most PAHs of interest.
Since exact densities of states are often unknown for interstellar PAHs, the
Whitten & Rabinovitch
(1963)
approximation, a semi-empirical expression,
has been extensively used in literature:
 (E) =
(E +
(E) =
(E + 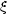 Ez)Nm-1 /
[(Nm - 1)!
Ez)Nm-1 /
[(Nm - 1)!
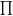 i=1Nm
h
i=1Nm
h i]
where
i]
where  (E)
is the density of states (the number of accessible vibrational states per
unit energy) at internal energy E,
Ez =
(E)
is the density of states (the number of accessible vibrational states per
unit energy) at internal energy E,
Ez = 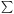 i=1Nm
(h
i=1Nm
(h i / 2)
is the total zero point energy of the molecule,
i / 2)
is the total zero point energy of the molecule,
 i is the
vibrational frequency,
and 0 <
i is the
vibrational frequency,
and 0 < 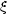 <
1 is an empirical correction factor.
<
1 is an empirical correction factor.
In contrast, the
Draine & Li (2001)
"exact-statistical" theory does not need this approximation; instead, they
calculate the "theoretical" mode spectrum from the Debye
model. A PAH molecule containing NC C atoms and
NH H atoms is treated by
Draine & Li (2001)
as having 5 different types of vibration:
(1) (NC - 2) out-of-plane C-C modes at
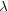 CC,op-1 = kB
CC,op-1 = kB
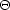 op /
hc
op /
hc  (16.7
µm)-1
(16.7
µm)-1
 600 cm-1
given by a two-dimensional Debye model with a Debye temperature
600 cm-1
given by a two-dimensional Debye model with a Debye temperature
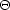 op
op
 950 K,
where kB is the Boltzmann constant,
(2) 2(NC - 2) in-plane C-C modes at
950 K,
where kB is the Boltzmann constant,
(2) 2(NC - 2) in-plane C-C modes at
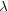 CC,ip-1 =
kB
CC,ip-1 =
kB
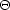 ip /
hc
ip /
hc  (5.7
µm)-1
(5.7
µm)-1
 1740 cm-1
given by a two-dimensional Debye model with a Debye temperature
1740 cm-1
given by a two-dimensional Debye model with a Debye temperature
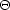 ip
ip
 2500 K,
(3) NH out-of-plane C-H bending modes at
2500 K,
(3) NH out-of-plane C-H bending modes at
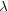 CH,op-1 = (11.3
µm)-1
CH,op-1 = (11.3
µm)-1
 886 cm-1,
(4) NH in-plane C-H bending modes at
886 cm-1,
(4) NH in-plane C-H bending modes at
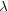 CH,ip-1 = (8.6
µm)-1
CH,ip-1 = (8.6
µm)-1
 1161 cm-1,
and (5) NH C-H stretching modes at
1161 cm-1,
and (5) NH C-H stretching modes at
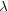 CH,str-1 = (3.3
µm)-1
CH,str-1 = (3.3
µm)-1
 3030 cm-1.
The "synthetic" mode spectrum for C24H12 is in
excellent agreement with the actual mode spectrum of coronene
(see Fig. 1 of
Draine & Li 2001).
Similarly, a silicate grain containing Na atoms is
treated as having
2 (Na - 2) vibrational modes distributed according to
a two-dimensional Debye model with a Debye temperature
3030 cm-1.
The "synthetic" mode spectrum for C24H12 is in
excellent agreement with the actual mode spectrum of coronene
(see Fig. 1 of
Draine & Li 2001).
Similarly, a silicate grain containing Na atoms is
treated as having
2 (Na - 2) vibrational modes distributed according to
a two-dimensional Debye model with a Debye temperature
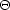 = 500 K,
and (Na - 2) modes described by
a three-dimensional Debye model with
= 500 K,
and (Na - 2) modes described by
a three-dimensional Debye model with
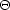 = 1500 K.
= 1500 K.
From the "synthetic" model mode spectrum we can obtain
the vibrational density of states and hence the "degeneracy"
gj, the number of distinct quantum states included in
bin j.
We note that the densities of states computed for
C24H12,
using both the actual normal mode spectrum for coronene
and the model normal mode spectrum for C24H12
(see Fig. 1 of
Draine & Li 2001)
are essentially identical for
E/hc 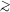 300 cm-1 (see Fig. 4 of
Draine & Li 2001).
With gj derived from the model mode spectrum,
and the j
300 cm-1 (see Fig. 4 of
Draine & Li 2001).
With gj derived from the model mode spectrum,
and the j
 i
(j < i)
excitation rates Tij calculated from
a known radiation field with energy density uE,
we can determine the i
i
(j < i)
excitation rates Tij calculated from
a known radiation field with energy density uE,
we can determine the i
 j
(i > j) de-excitation transition rates
Tji.
Solving the steady-state state probability evolution equation
j
(i > j) de-excitation transition rates
Tji.
Solving the steady-state state probability evolution equation
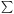 j
j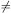 i
TijPj =
i
TijPj =
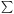 j
j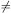 i
Tji Pi
for i = 0, 1,..., M, we are able to obtain the steady-state
energy probability distribution Pj
and calculate the resulting IR emission spectrum.
i
Tji Pi
for i = 0, 1,..., M, we are able to obtain the steady-state
energy probability distribution Pj
and calculate the resulting IR emission spectrum.
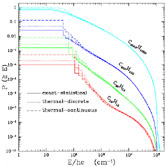 |
Figure 4. The cumulative energy probability
distributions for PAHs illuminated by the general ISRF
computed using the exact-statistical model,
the thermal-discrete model,
and the thermal-continuous model.
Note that the lowest energy state (E = 0), not shown here,
has P(E |
In Figure 4 we present the cumulative
energy probability distributions for selected PAHs excited by
the general solar neighbourhood
interstellar starlight radiation field (ISRF) of
Mathis, Mezger & Panagia
(1983, hereafter MMP)
obtained from the "exact-statistical" model.
It is seen in Figure 4 that the probability of
being in the ground state is very large for small grains:
for example, for the MMP radiation field,
grains with NC
 4000 spend most
of their time at E = 0. The sharp drop at 13.6eV (E /
hc
4000 spend most
of their time at E = 0. The sharp drop at 13.6eV (E /
hc  1.1 × 105cm-1)
is due to the radiation field cutoff at 912Å and
to the fact that multiphoton events are rare.
The resulting IR emission spectra are displayed in
Figure 5. The sawtooth features seen at long
wavelengths
are due to our treatment of transitions from the lower excited
energy bins to the ground state and first few excited states
(see Section 5.1 and Appendix B of
Draine & Li 2001).
1.1 × 105cm-1)
is due to the radiation field cutoff at 912Å and
to the fact that multiphoton events are rare.
The resulting IR emission spectra are displayed in
Figure 5. The sawtooth features seen at long
wavelengths
are due to our treatment of transitions from the lower excited
energy bins to the ground state and first few excited states
(see Section 5.1 and Appendix B of
Draine & Li 2001).
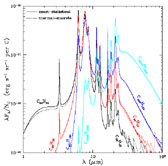 |
Figure 5. IR emissivities (per C atom) for selected ionized PAHs in the general ISRF calculated using the exact-statistical and thermal-discrete models. Taken from Draine & Li (2001). |