


A bound electron in an ion can be brought into a higher, excited energy level through a collision with a free electron or by absorption of a photon. The latter will be discussed in more detail in Sect. 6. Here we focus upon excitation by electrons.
The cross section Qij for excitation from level i to level j for this process can be conveniently parametrised by
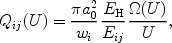 |
(1) |
where U = Eij / E with
Eij the excitation energy from level i to
j, E the energy of the exciting electron,
EH the Rydberg
energy (13.6 eV), a0 the Bohr radius and
wi the statistical weight
of the lower level i. The dimensionless quantity
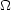 (U) is the so-called
collision strength. For a given transition on an iso-electronic sequence,
(U) is the so-called
collision strength. For a given transition on an iso-electronic sequence,
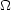 (U) is not a
strong function of the atomic number
Z, or may be even almost independent of Z.
(U) is not a
strong function of the atomic number
Z, or may be even almost independent of Z.
Mewe (1972) introduced a convenient formula that can be used to describe most collision strengths, written here as follows:
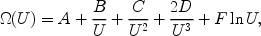 |
(2) |
where A, B, C, D and F are parameters that differ for each transition. The expression can be integrated analytically over a Maxwellian electron distribution, and the result can be expressed in terms of exponential integrals. We only mention here that the total excitation rate Sij (in units of m-3 s-1) is given by
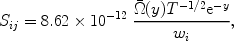 |
(3) |
with y  Eij / kT and
Eij / kT and
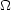 (y) is the
Maxwellian-averaged collision strength. For low temperatures, y
>> 1 and
(y) is the
Maxwellian-averaged collision strength. For low temperatures, y
>> 1 and
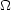 (y) =
A + B + C + 2D, leading
to Sij ~ T-1/2
e-y. The excitation rate drops exponentially due to
the lack of electrons with sufficient
energy. In the other limit of high temperature, y << 1 and
(y) =
A + B + C + 2D, leading
to Sij ~ T-1/2
e-y. The excitation rate drops exponentially due to
the lack of electrons with sufficient
energy. In the other limit of high temperature, y << 1 and
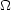 (y) =
-F lny and hence
Sij ~ T-1/2 lny.
(y) =
-F lny and hence
Sij ~ T-1/2 lny.
Not all transitions have this asymptotic behaviour, however. For instance, so-called forbidden transitions have F = 0 and hence have much lower excitation rates at high energy. So-called spin-forbidden transitions even have A = B = F = 0.
In most cases, the excited state is stable and the ion will decay back to the ground level by means of a radiative transition, either directly or through one or more steps via intermediate energy levels. Only in cases of high density or high radiation fields, collisional excitation or further radiative excitation to a higher level may become important, but for typical cluster and ISM conditions these processes are not important in most cases. Therefore, the excitation rate immediately gives the total emission line power.
Collisional ionisation occurs when during the interaction of a free
electron with an atom or ion the free electron transfers a part of its
energy to one of the bound electrons, which is then able to escape from
the ion. A necessary condition is that the kinetic energy E of
the free electron must be larger than the binding energy I of the
atomic shell from which the bound electron escapes. A formula that gives
a correct order of magnitude estimate of the cross section
 of
this process and that has the proper asymptotic behaviour (first
calculated by Bethe and Born) is the formula of
Lotz (1968):
of
this process and that has the proper asymptotic behaviour (first
calculated by Bethe and Born) is the formula of
Lotz (1968):
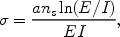 |
(4) |
where ns is the number of electrons in the shell and the normalisation a = 4.5 × 10-24 m2 keV2. This equation shows that high-energy electrons have less ionising power than low-energy electrons. Also, the cross section at the threshold E = I is zero.
The above cross section has to be averaged over the electron distribution (Maxwellian for a thermal plasma). For simple formulae for the cross section such as (4) the integration can be done analytically and the result can be expressed in terms of exponential integrals. We give here only the asymptotic results for CDI, the total number of direct ionisations per unit volume per unit time:
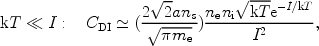 |
(5) |
and
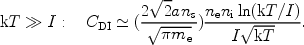 |
(6) |
For low temperatures, the ionisation rate therefore goes exponentially to zero. This can be understood simply, because for low temperatures only the electrons from the exponential tail of the Maxwell distribution have sufficient energy to ionise the atom or ion. For higher temperatures the ionisation rate also approaches zero, but this time because the cross section at high energies is small.
For each ion the direct ionisation rate per atomic shell can now be determined. The total rate follows immediately by adding the contributions from the different shells. Usually only the outermost two or three shells are important. That is because of the scaling with I-2 and I-1 in (5) and (6), respectively.
This process is very similar to collisional ionisation. The difference is that in the case of photoionisation a photon instead of an electron is causing the ionisation. Further, the effective cross section differs from that for collisional ionisation. As an example Fig. 2 shows the cross section for neutral iron and Na-like iron. For the more highly ionised iron the so-called "edges" (corresponding to the ionisation potentials I) occur at higher energies than for neutral iron. Contrary to the case of collisional ionisation, the cross section at threshold for photoionisation is not zero. The effective cross section just above the edges sometimes changes rapidly (the so-called Cooper minima and maxima).
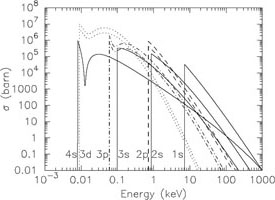 |
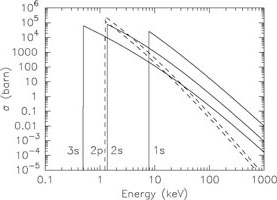 |
Figure 2. Photoionisation cross section in barn (10-28 m-2) for Fe I (left) and Fe XVI (right). The p and d states (dashed and dotted) have two lines each because of splitting into two sublevels with different j (see Sect. 2.1). |
|
Contrary to collisional ionisation, all the inner shells now have the largest cross section. For the K-shell one can approximate for E > I
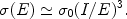 |
(7) |
For a given ionising spectrum F(E) (photons per unit volume per unit energy) the total number of photoionisations follows as
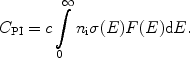 |
(8) |
For hydrogenlike ions one can write:
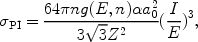 |
(9) |
where n is the principal quantum number,
 the fine structure constant
and a0 the Bohr radius. The Gaunt factor
g(E, n) is of order unity and
varies only slowly as a function of E. It has been calculated and
tabulated by
Karzas &
Latter (1961).
The above equation is also applicable to
excited states of the atom, and is a good approximation for all excited
atoms or ions where n is larger than the corresponding value for
the valence electron.
the fine structure constant
and a0 the Bohr radius. The Gaunt factor
g(E, n) is of order unity and
varies only slowly as a function of E. It has been calculated and
tabulated by
Karzas &
Latter (1961).
The above equation is also applicable to
excited states of the atom, and is a good approximation for all excited
atoms or ions where n is larger than the corresponding value for
the valence electron.
Scattering of a photon on an electron generally leads to energy transfer from one of the particles to the other. In most cases only scattering on free electrons is considered. But Compton scattering also can occur on bound electrons. If the energy transfer from the photon to the electron is large enough, the ionisation potential can be overcome leading to ionisation. This is the Compton ionisation process.
In the Thomson limit the differential cross section for Compton scattering is given by
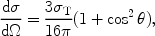 |
(10) |
with 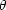 the scattering angle and
the scattering angle and
 T the Thomson
cross section (6.65 × 10-29 m-2). The energy
transfer
T the Thomson
cross section (6.65 × 10-29 m-2). The energy
transfer  E
during the scattering is given by (E is the photon energy):
E
during the scattering is given by (E is the photon energy):
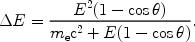 |
(11) |
Only those scatterings where
 E >
I contribute to the ionisation. This
defines a critical angle
E >
I contribute to the ionisation. This
defines a critical angle 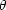 c, given by:
c, given by:
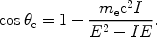 |
(12) |
For E >> I we have
 (E)
(E)

 T
(all scatterings
lead in that case to ionisation) and further for
T
(all scatterings
lead in that case to ionisation) and further for
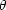 c
c

 we have
we have
 (E)
(E)
 0. Because for most ions I <<
me c2, this last condition occurs
for E
0. Because for most ions I <<
me c2, this last condition occurs
for E 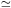 (Imec2 / 2)1/2 >>
I. See Fig. 3 for an example of some cross
sections. In general, Compton ionisation is important if the ionising
spectrum contains a significant hard X-ray contribution, for which the
Compton cross section is larger than the steeply falling photoionisation
cross section.
(Imec2 / 2)1/2 >>
I. See Fig. 3 for an example of some cross
sections. In general, Compton ionisation is important if the ionising
spectrum contains a significant hard X-ray contribution, for which the
Compton cross section is larger than the steeply falling photoionisation
cross section.
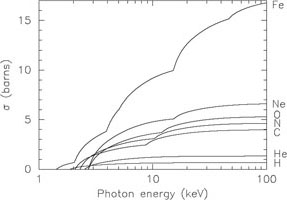 |
Figure 3. Compton ionisation cross section for neutral atoms of H, He, C, N, O, Ne and Fe. |
3.2.4. Autoionisation and fluorescence
As we showed above, interaction of a photon or free electron with an atom or ion may lead to ionisation. In particular when an electron from one of the inner shells is removed, the resulting ion has a "vacancy" in its atomic structure and is unstable. Two different processes may occur to stabilise the ion again.
The first process is fluorescence. Here one of the electrons from the outer shells makes a radiative transition in order to occupy the vacancy. The emitted photon has an energy corresponding to the energy difference between the initial and final discrete states.
The other possibility to fill the gap is auto-ionisation through the Auger process. In this case, also one of the electrons from the outer shells fills the vacancy in the lower level. The released energy is not emitted as a photon, however, but transferred to another electron from the outer shells that is therefore able to escape from the ion. As a result, the initial ionisation may lead to a double ionisation. If the final electron configuration of the ion still has holes, more auto-ionisations or fluorescence may follow until the ion has stabilised.
In Fig. 4 the fluorescence yield
 (probability that a vacancy will be filled by a radiative transition) is
shown for all elements. In general, the fluorescence yield increases
strongly with increasing nuclear charge Z, and is higher for the
innermost atomic shells. As a typical example, for Fe I a K-shell
vacancy has
(probability that a vacancy will be filled by a radiative transition) is
shown for all elements. In general, the fluorescence yield increases
strongly with increasing nuclear charge Z, and is higher for the
innermost atomic shells. As a typical example, for Fe I a K-shell
vacancy has  = 0.34,
while an Li-shell vacancy has
= 0.34,
while an Li-shell vacancy has
 = 0.001. For O I these
numbers are 0.009 and 0, respectively.
= 0.001. For O I these
numbers are 0.009 and 0, respectively.
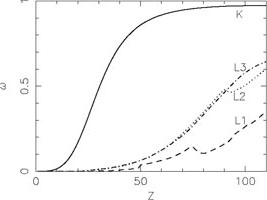 |
 |
Figure 4. Left panel:
Fluorescence yield
|
|
3.2.5. Excitation-Autoionisation
In Sect. 3.2.1 we showed how the collision of a free electron with an ion can lead to ionisation. In principle, if the free electron has insufficient energy (E < I), there will be no ionisation. However, even in that case it is sometimes still possible to ionise, but in a more complicated way. The process is as follows. The collision can bring one of the electrons in the outermost shells in a higher quantum level (excitation). In most cases, the ion will return to its ground level by a radiative transition. But in some cases the excited state is unstable, and a radiationless Auger transition can occur (see Sect. 3.2.4). The vacancy that is left behind by the excited electron is being filled by another electron from one of the outermost shells, while the excited electron is able to leave the ion (or a similar process with the role of both electrons reversed).
Because of energy conservation, this process only occurs if the excited electron comes from one of the inner shells (the ionisation potential barrier must be taken anyhow). The process is in particular important for Li-like and Na-like ions, and for several other individual atoms and ions. As an example we treat here Li-like ions (see Fig. 5, left panel). In that case the most important contribution comes from a 1s-2p excitation.
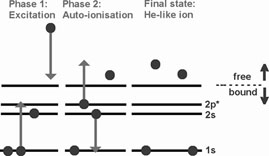 |
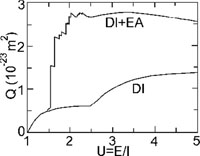 |
Figure 5. Left panel: the Excitation-Autoionisation process for a Li-like ion. Right panel: collisional ionisation cross section for Fe XVI. The contribution from direct collisional ionisation (DI) and excitation-autoionisation (EA) are indicated. Adapted from Arnaud & Rothenflug (1985). |
|
3.3.1. Radiative recombination
Radiative recombination is the reverse process of photoionisation. A free
electron is captured by an ion while emitting a photon. The released
radiation is the so-called free-bound continuum emission. It is
relatively easy to show that there is a simple relation between the
photoionisation cross section
 bf(E)
and the recombination cross
section
bf(E)
and the recombination cross
section  fb,
namely the Milne-relation:
fb,
namely the Milne-relation:
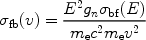 |
(13) |
where gn is the statistical weight of the quantum level into which the electron is captured (for an empty shell this is gn = 2n2). By averaging over a Maxwell distribution one gets the recombination-coefficient to level n:
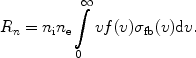 |
(14) |
Of course there is energy conservation, so E = 1/2 me v2 + I.
It can be shown that for the photoionisation cross section (9) and for g = 1, constant and gn = 2n2:
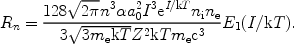 |
(15) |
With the asymptotic relations for the exponential integrals it can be shown that
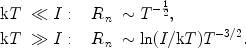 |
(16) (17) |
Therefore for T  0 the recombination coefficient approaches
infinity: a cool plasma is hard to ionise. For T
0 the recombination coefficient approaches
infinity: a cool plasma is hard to ionise. For T

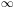 the
recombination coefficient goes to zero, because of the Milne relation
(v
the
recombination coefficient goes to zero, because of the Milne relation
(v
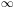 )
and because of the sharp decrease of the
photoionisation cross section for high energies.
)
and because of the sharp decrease of the
photoionisation cross section for high energies.
As a rough approximation we can use further that I ~
(Z / n)2. Substituting
this we find that for kT << I (recombining plasmas)
Rn ~ n-1, while for kT
>> I (ionising plasmas) Rn ~
n-3. In
recombining plasmas in particular many higher excited levels will be
populated by the recombination, leading to significantly stronger line
emission. On the other hand, in ionising plasmas (such as supernova
remnants) recombination mainly occurs to the lowest levels. Note that
for recombination to the ground level the approximation (15) cannot be
used (the hydrogen limit), but instead one should use the exact
photoionisation cross section of the valence electron. By adding over
all values of n and applying an approximation
Seaton (1959)
found for the total radiative recombination rate
 RR (in units
of m-3 s-1):
RR (in units
of m-3 s-1):
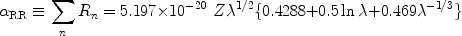 |
(18) |
with 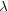
 EH Z2 /
kT and EH the Rydberg energy (13.6 eV). Note
that this equation only holds for hydrogen-like ions. For other ions
usually an analytical fit to numerical calculations is used:
EH Z2 /
kT and EH the Rydberg energy (13.6 eV). Note
that this equation only holds for hydrogen-like ions. For other ions
usually an analytical fit to numerical calculations is used:
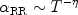 |
(19) |
where the approximation is strictly speaking only valid for T
near the equilibrium concentration. The approximations (16) and
(17) raise suspicion that for T
 0 or T
0 or T

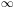 (19) could be a poor
choice.
(19) could be a poor
choice.
The captured electron does not always reach the ground level immediately. We have seen before that in particular for cool plasmas (kT << I) the higher excited levels are frequently populated. In order to get to the ground state, one or more radiative transitions are required. Apart from cascade corrections from and to higher levels the recombination line radiation is essentially given by (15). A comparison of recombination with excitation tells that in particular for low temperatures (compared to the line energy) recombination radiation dominates, and for high temperatures excitation radiation dominates. This is also one of the main differences between photoionised and collisionally ionised plasmas, as photoionised plasmas in general have a low temperature compared to the typical ionisation potentials.
3.3.2. Dielectronic recombination
This process is more or less the inverse of excitation-autoionisation. Now a
free electron interacts with an ion, by which it is caught (quantum level
n"  ") but
at the same time it excites an
electron from (n
") but
at the same time it excites an
electron from (n )
)
 (n'
(n'
 '). The doubly excited
state is in general not stable, and the ion will return to its original
state by
auto-ionisation. However there is also a possibility that one of the excited
electrons (usually the electron that originally belonged to the ion)
falls back
by a radiative transition to the ground level, creating therefore a stable,
albeit excited state (n"
'). The doubly excited
state is in general not stable, and the ion will return to its original
state by
auto-ionisation. However there is also a possibility that one of the excited
electrons (usually the electron that originally belonged to the ion)
falls back
by a radiative transition to the ground level, creating therefore a stable,
albeit excited state (n"
 ") of the ion. In
particular excitations with
") of the ion. In
particular excitations with  '
=
'
=  + 1 contribute much to this
process. In order to calculate this process, one should take account of many
combinations (n'
+ 1 contribute much to this
process. In order to calculate this process, one should take account of many
combinations (n'
 ')(n"
')(n"
 ").
").
The final transition probability is often approximated by
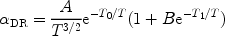 |
(20) |
where A, B, T0 and T1
are adjustable parameters. Note that for T

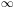 the
asymptotic behaviour is identical to the case of
radiative recombination. For T
the
asymptotic behaviour is identical to the case of
radiative recombination. For T
 0 however, dielectronic
recombination can be neglected; this is because the free electron has
insufficient energy to excite a bound electron. Dielectronic
recombination is a dominant process in the Solar corona, and also in
other situations it is often very important.
0 however, dielectronic
recombination can be neglected; this is because the free electron has
insufficient energy to excite a bound electron. Dielectronic
recombination is a dominant process in the Solar corona, and also in
other situations it is often very important.
Dielectronic recombination produces more than one line photon. Consider for example the dielectronic recombination of a He-like ion into a Li-like ion:
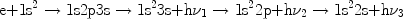 |
(21) |
The first arrow corresponds to the electron capture, the second arrow to the
stabilising radiative transition 2p
 1s and the third arrow
to the radiative transition 3s
1s and the third arrow
to the radiative transition 3s
 2p of the captured
electron. This last
transition would have also occurred if the free electron was caught
directly into the 3s shell by normal radiative recombination. Finally,
the electron has
to decay further to the ground level and this can go through the normal
transitions in a Li-like ion (fourth arrow). This single recombination thus
produces three line photons.
2p of the captured
electron. This last
transition would have also occurred if the free electron was caught
directly into the 3s shell by normal radiative recombination. Finally,
the electron has
to decay further to the ground level and this can go through the normal
transitions in a Li-like ion (fourth arrow). This single recombination thus
produces three line photons.
Because of the presence of the extra electron in the higher orbit, the
energy h 1 of
the 2p
1 of
the 2p
 1s transition is
slightly different from the energy in a normal He-like ion. The
stabilising transition is therefore also called a satellite
line. Because there are many different possibilities for the orbit of
the captured electron, one usually finds a forest of such satellite
lines surrounding the normal 2p
1s transition is
slightly different from the energy in a normal He-like ion. The
stabilising transition is therefore also called a satellite
line. Because there are many different possibilities for the orbit of
the captured electron, one usually finds a forest of such satellite
lines surrounding the normal 2p
 1s excitation line in
the He-like ion (or analogously for other iso-electronic sequences).
Fig. 6 gives an example of these satellite lines.
1s excitation line in
the He-like ion (or analogously for other iso-electronic sequences).
Fig. 6 gives an example of these satellite lines.
3.4. Charge transfer processes
In most cases ionisation or recombination in collisionally ionised plasmas is caused by interactions of an ion with a free electron. At low temperatures (typically below 105 K) also charge transfer reactions become important. During the interaction of two ions, an electron may be transferred from one ion to the other; it is usually captured in an excited state, and the excited ion is stabilised by one or more radiative transitions. As hydrogen and helium outnumber by at least a factor of 100 any other element (see Table 3), in practice only interactions between those elements and heavier nuclei are important. Reactions with H I and He I lead to recombination of the heavier ion, and reactions with H II and He II to ionisation.
The electron captured during charge transfer recombination of an oxygen ion (for instance O VII, O VIII) is usually captured in an intermediate quantum state (principal quantum number n = 4-6). This leads to enhanced line emission from that level as compared to the emission from other principal levels, and this signature can be used to show the presence of charge transfer reactions. Another signature - actually a signature for all recombining plasmas - is of course the enhancement of the forbidden line relative to the resonance line in the O VII triplet (Sect. 5.2.2).
An important example is the charge transfer of highly charged ions from the Solar wind with the neutral or weakly ionised Geocorona. Whenever the Sun is more active, this process may produce enhanced thermal soft X-ray emission in addition to the steady foreground emission from our own Galaxy. See Bhardwaj et al. (2006) for a review of X-rays from the Solar System. Care should be taken not to confuse this temporary enhanced Geocoronal emission with soft excess emission in a background astrophysical source.