


5.1. Continuum emission processes
In any plasma there are three important continuum emission processes, that we briefly mention here: Bremsstrahlung, free-bound emission and two-photon emission.
Bremsstrahlung is caused by a collision between a free electron and an
ion. The emissivity
 ff
(photons m-3 s-1 J-1) can be written as:
ff
(photons m-3 s-1 J-1) can be written as:
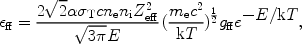 |
(29) |
where  is the fine
structure constant,
is the fine
structure constant,
 T the
Thomson cross section, ne and ni the
electron and ion density, and E the energy of the emitted
photon. The factor gff
is the so-called Gaunt factor and is a dimensionless quantity of order
unity. Further, Zeff is the effective charge of the
ion, defined as
T the
Thomson cross section, ne and ni the
electron and ion density, and E the energy of the emitted
photon. The factor gff
is the so-called Gaunt factor and is a dimensionless quantity of order
unity. Further, Zeff is the effective charge of the
ion, defined as
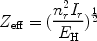 |
(30) |
where EH is the ionisation energy of hydrogen (13.6 eV), Ir the ionisation potential of the ion after a recombination, and nr the corresponding principal quantum number.
It is also possible to write (29) as
 ff =
Pff ne ni with
ff =
Pff ne ni with
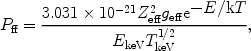 |
(31) |
where in this case Pff is in photons × m3s-1 keV-1 and EkeV is the energy in keV. The total amount of radiation produced by this process is given by
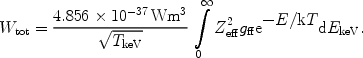 |
(32) |
From (29) we see immediately that the Bremsstrahlung spectrum
(expressed in W m-3 keV-1) is flat for E
<< kT, and for
E > kT it drops exponentially. In order to measure the
temperature of a hot plasma, one needs to measure near E
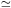 kT. The Gaunt factor
gff can be calculated analytically; there are both
tables and asymptotic approximations available. In general,
gff depends on
both E / kT and kT / Zeff.
kT. The Gaunt factor
gff can be calculated analytically; there are both
tables and asymptotic approximations available. In general,
gff depends on
both E / kT and kT / Zeff.
For a plasma (29) needs to be summed over all ions that are present in order to calculate the total amount of Bremsstrahlung radiation. For cosmic abundances, hydrogen and helium usually yield the largest contribution. Frequently, one defines an average Gaunt factor Gff by
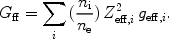 |
(33) |
Free-bound emission occurs during radiative recombination (Sect. 3.3.1). The energy of the emitted photon is at least the ionisation energy of the recombined ion (for recombination to the ground level) or the ionisation energy that corresponds to the excited state (for recombination to higher levels). From the recombination rate (see Sect. 3.3.1) the free-bound emission is determined immediately:
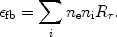 |
(34) |
Also here it is possible to define an effective Gaunt factor Gfb. Free-bound emission is in practice often very important. For example in CIE for kT = 0.1 keV, free-bound emission is the dominant continuum mechanism for E > 0.1 keV; for kT = 1 keV it dominates above 3 keV. For kT >> 1 keV Bremsstrahlung is always the most important mechanism, and for kT << 0.1 keV free-bound emission dominates. See also Fig. 8.
Of course, under conditions of photoionisation equilibrium free-bound emission is even more important, because there are more recombinations than in the CIE case (because T is lower, at comparable ionisation).
This process is in particular important for hydrogen-like or helium-like ions. After a collision with a free electron, an electron from a bound 1s shell is excited to the 2s shell. The quantum-mechanical selection rules do not allow that the 2s electron decays back to the 1s orbit by a radiative transition. Usually the ion will then be excited to a higher level by another collision, for example from 2s to 2p, and then it can decay radiatively back to the ground state (1s). However, if the density is very low (ne << ne, crit, Eqn. 36 - 37), the probability for a second collision is very small and in that case two-photon emission can occur: the electron decays from the 2s orbit to the 1s orbit while emitting two photons. Energy conservation implies that the total energy of both photons should equal the energy difference between the 2s and 1s level (E2phot = E1s - E2s). From symmetry considerations it is clear that the spectrum must be symmetrical around E = 0.5E2phot, and further that it should be zero for E = 0 and E = E2phot. An empirical approximation for the shape of the spectrum is given by:
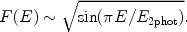 |
(35) |
An approximation for the critical density below which two photon emission is important can be obtained from a comparison of the radiative and collisional rates from the upper (2s) level, and is given by (Mewe et al., 1986):
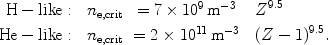 |
(36) (37) |
For example for carbon two photon emission is important for densities below
1017 m-3, which is the case for many astrophysical
applications. Also in this case one can determine an average Gaunt
factor G2phot by
averaging over all ions. Two photon emission is important in practice for
0.5 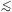 kT
kT
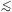 5 keV, and then
in particular for the contributions of
C, N and O between 0.2 and 0.6 keV. See also Fig. 8.
5 keV, and then
in particular for the contributions of
C, N and O between 0.2 and 0.6 keV. See also Fig. 8.
Apart from continuum radiation, line radiation plays an important role for thermal plasmas. In some cases the flux, integrated over a broad energy band, can be completely dominated by line radiation (see Fig. 8). The production process of line radiation can be broken down in two basic steps: the excitation and the emission process.
An atom or ion must first be brought into an excited state before it can emit line photons. There are several physical processes that can contribute to this.
The most important process is usually collisional excitation (Sect. 3.1), in particular for plasmas in CIE. The collision of an electron with the ion brings it in an excited state.
A second way to excite the ion is by absorbing a photon with the proper energy. We discuss this process in more detail in Sect. 6.
Alternatively, inner shell ionisation (either by the collision with a free electron, Sect. 3.2.1 or by the photoelectric effect, Sect. 3.2.2) brings the ion in an excited state.
Finally, the ion can also be brought in an excited state by capturing a free electron in one of the free energy levels above the ground state (radiative recombination, Sect. 3.3.1), or through dielectronic recombination (Sect 3.3.2).
It does not matter by whatever process the ion is brought into an excited state j, whenever it is in such a state it may decay back to the ground state or any other lower energy level i by emitting a photon. The probability per unit time that this occurs is given by the spontaneous transition probability Aij (units: s-1) which is a number that is different for each transition. The total line power Pij (photons per unit time and volume) is then given by
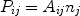 |
(38) |
where nj is the number density of ions in the excited state j. For the most simple case of excitation from the ground state g (rate Sgj) followed by spontaneous emission, one can simply approximate ng neSgj = nj Agj. From this equation, the relative population nj / ng << 1 is determined, and then using (38) the line flux is determined. In realistic situations, however, things are more complicated. First, the excited state may also decay to other intermediate states if present, and also excitations or cascades from other levels may play a role. Furthermore, for high densities also collisional excitation or de-excitation to and from other levels becomes important. In general, one has to solve a set of equations for all energy levels of an ion where all relevant population and depopulation processes for that level are taken into account. For the resulting solution vector nj, the emitted line power is then simply given by Eqn. (38).
Note that not all possible transitions between the different energy levels are allowed. There are strict quantum mechanical selection rules that govern which lines are allowed; see for instance Herzberg (1944) or Mewe (1999). Sometimes there are higher order processes that still allow a forbidden transition to occur, albeit with much smaller transition probabilities Aij. But if the excited state j has no other (fast enough) way to decay, these forbidden lines occur and the lines can be quite strong, as their line power is essentially governed by the rate at which the ion is brought into its excited state j.
One of the most well known groups of lines is the He-like 1s-2p triplet. Usually the strongest line is the resonance line, an allowed transition. The forbidden line has a similar strength as the resonance line, for the low density conditions in the ISM and intracluster medium, but it can be relatively enhanced in recombining plasmas, or relatively reduced in high density plasmas like stellar coronal loops. In between both lines is the intercombination line. In fact, this intercombination line is a doublet but for the lighter elements both components cannot be resolved. But see Fig. 6 for the case of iron.
For most X-ray spectral lines, the line profile of a line with energy
E can be approximated with a Gaussian
exp(- E2 /
2
E2 /
2 2) with
2) with
 given by
given by
 / E =
/ E =
 v / c
where the velocity dispersion is
v / c
where the velocity dispersion is
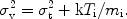 |
(39) |
Here Ti is the ion temperature (not necessarily the
same as the electron temperature), and
 t is the
root mean squared turbulent velocity of the emitting medium. For large
ion temperature, turbulent velocity or high spectral resolution this
line width can be measured, but in most cases the lines are not resolved
for CCD type spectra.
t is the
root mean squared turbulent velocity of the emitting medium. For large
ion temperature, turbulent velocity or high spectral resolution this
line width can be measured, but in most cases the lines are not resolved
for CCD type spectra.
Resonance scattering is a process where a photon is absorbed by an atom and then re-emitted as a line photon of the same energy into a different direction. As for strong resonance lines (allowed transitions) the transition probabilities Aij are large, the time interval between absorption and emission is extremely short, and that is the reason why the process effectively can be regarded as a scattering process. We discuss the absorption properties in Sect. 6.3, and have already discussed the spontaneous emission in Sect. 5.2.2.
Resonance scattering of X-ray photons is potentially important in the dense cores of some clusters of galaxies for a few transitions of the most abundant elements, as first shown by Gil'fanov et al. (1987). The optical depth for scattering can be written conveniently as (cf. also Sect. 6.3):
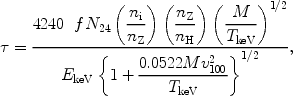 |
(40) |
where f is the absorption oscillator strength of the line (of order unity for the strongest spectral lines), EkeV the energy in keV, N24 the hydrogen column density in units of 1024 m-2, ni the number density of the ion, nz the number density of the element, M the atomic weight of the ion, TkeV the ion temperature in keV (assumed to be equal to the electron temperature) and v100 the micro-turbulence velocity in units of 100 km/s. Resonance scattering in clusters causes the radial intensity profile on the sky of an emission line to become weaker in the cluster core and stronger in the outskirts, without destroying photons. By comparing the radial line profiles of lines with different optical depth, for instance the 1s-2p and 1s-3p lines of O VII or Fe XXV, one can determine the optical depth and hence constrain the amount of turbulence in the cluster.
Another important application was proposed by Churazov et al. (2001). They show that for WHIM filaments the resonance line of the O VII triplet can be enhanced significantly compared to the thermal emission of the filament due to resonant scattering of X-ray background photons on the filament. The ratio of this resonant line to the other lines of the triplet therefore can be used to estimate the column density of a filament.
5.3. Some important line transitions
In Tables 4 - 5 we list the 100 strongest emission lines under CIE conditions. Note that each line has its peak emissivity at a different temperature. In particular some of the H-like and He-like transitions are strong, and further the so-called Fe-L complex (lines from n = 2 in Li-like to Ne-like iron ions) is prominent. At longer wavelengths, the L-complex of Ne Mg, Si and S gives strong soft X-ray lines. At very short wavelengths, there are not many strong emission lines: between 6-7 keV, the Fe-K emission lines are the strongest spectral features.
| E |  |
-log | log | ion | iso-el. | lower | upper |
| (eV) | (Å) | Qmax | Tmax | seq. | level | level | |
| 126.18 | 98.260 | 1.35 | 5.82 | Ne VIII | Li | 2p 2P3/2 | 3d 2D5/2 |
| 126.37 | 98.115 | 1.65 | 5.82 | Ne VIII | Li | 2p 2P1/2 | 3d 2D3/2 |
| 127.16 | 97.502 | 1.29 | 5.75 | Ne VII | Be | 2s 1S0 | 3p 1P1 |
| 128.57 | 96.437 | 1.61 | 5.47 | Si V | Ne | 2p 1S0 | 3d 1P1 |
| 129.85 | 95.483 | 1.14 | 5.73 | Mg VI | N | 2p 4S3/2 | 3d 4P5/2,3/2,1/2 |
| 132.01 | 93.923 | 1.46 | 6.80 | Fe XVIII | F | 2s 2P3/2 | 2p 2S1/2 |
| 140.68 | 88.130 | 1.21 | 5.75 | Ne VII | Be | 2p 3P1 | 4d 3D2,3 |
| 140.74 | 88.092 | 1.40 | 5.82 | Ne VIII | Li | 2s 2S1/2 | 3p 2P3/2 |
| 147.67 | 83.959 | 0.98 | 5.86 | Mg VII | C | 2p 3P | 3d 3D, 1D, 3F |
| 148.01 | 83.766 | 1.77 | 5.86 | Mg VII | C | 2p 3P2 | 3d 3P2 |
| 148.56 | 83.457 | 1.41 | 5.90 | Fe IX | Ar | 3p 1S0 | 4d 3P1 |
| 149.15 | 83.128 | 1.69 | 5.69 | Si VI | F | 2p 2P3/2 | (3P)3d 2D5/2 |
| 149.38 | 83.000 | 1.48 | 5.74 | Mg VI | N | 2p4 S3/2 | 4d4 P5/2,3/2,1/2 |
| 150.41 | 82.430 | 1.49 | 5.91 | Fe IX | Ar | 3p 1S0 | 4d 1P1 |
| 154.02 | 80.501 | 1.75 | 5.70 | Si VI | F | 2p 2P3/2 | (1D)3d 2D5/2 |
| 159.23 | 77.865 | 1.71 | 6.02 | Fe X | Cl | 3p 2P1/2 | 4d 2D5/2 |
| 165.24 | 75.034 | 1.29 | 5.94 | Mg VIII | B | 2p 2P3/2 | 3d 2D5/2 |
| 165.63 | 74.854 | 1.29 | 5.94 | Mg VIII | B | 2p 2P1/2 | 3d 2D3/2 |
| 170.63 | 72.663 | 1.07 | 5.76 | S VII | Ne | 2p 1S0 | 3s 3P1,2 |
| 170.69 | 72.635 | 1.61 | 6.09 | Fe XI | S | 3p 3P2 | 4d 3D3 |
| 171.46 | 72.311 | 1.56 | 6.00 | Mg IX | Be | 2p 1P1 | 3d 1D2 |
| 171.80 | 72.166 | 1.68 | 6.08 | Fe XI | S | 3p 1D2 | 4d 1F3 |
| 172.13 | 72.030 | 1.44 | 6.00 | Mg IX | Be | 2p 3P2,1 | 3s 3S1 |
| 172.14 | 72.027 | 1.40 | 5.76 | S VII | Ne | 2p 1S0 | 3s 1P1 |
| 177.07 | 70.020 | 1.18 | 5.84 | Si VII | O | 2p 3P | 3d 3D, 3P |
| 177.98 | 69.660 | 1.36 | 6.34 | Fe XV | Mg | 3p 1P1 | 4s 1S0 |
| 177.99 | 69.658 | 1.57 | 5.96 | Si VIII | N | 2p 4S3/2 | 3s 4P5/2,3/2,1/2 |
| 179.17 | 69.200 | 1.61 | 5.71 | Si VI | F | 2p 2P | 4d 2P, 2D |
| 186.93 | 66.326 | 1.72 | 6.46 | Fe XVI | Na | 3d 2D | 4f 2F |
| 194.58 | 63.719 | 1.70 | 6.45 | Fe XVI | Na | 3p 2P3/2 | 4s 2S1/2 |
| 195.89 | 63.294 | 1.64 | 6.08 | Mg X | Li | 2p 2P3/2 | 3d 2D5/2 |
| 197.57 | 62.755 | 1.46 | 6.00 | Mg IX | Be | 2s 1S0 | 3p 1P1 |
| 197.75 | 62.699 | 1.21 | 6.22 | Fe XIII | Si | 3p 3P1 | 4d 3D2 |
| 198.84 | 62.354 | 1.17 | 6.22 | Fe XIII | Si | 3p 3P0 | 4d 3D1 |
| 199.65 | 62.100 | 1.46 | 6.22 | Fe XIII | Si | 3p 3P1 | 4d 3P0 |
| 200.49 | 61.841 | 1.29 | 6.07 | Si IX | C | 2p 3P2 | 3s 3P1 |
| 203.09 | 61.050 | 1.06 | 5.96 | Si VIII | N | 2p 4S3/2 | 3d 4P5/2,3/2,1/2 |
| 203.90 | 60.807 | 1.69 | 5.79 | S VII | Ne | 2p 1S0 | 3d 3D1 |
| 204.56 | 60.610 | 1.30 | 5.79 | S VII | Ne | 2p 1S0 | 3d 1P1 |
| 223.98 | 55.356 | 1.00 | 6.08 | Si IX | C | 2p 3P | 3d 3D, 1D, 3F |
| 234.33 | 52.911 | 1.34 | 6.34 | Fe XV | Mg | 3s 1S0 | 4p 1P1 |
| 237.06 | 52.300 | 1.61 | 6.22 | Si XI | Be | 2p 1P1 | 3s 1S0 |
| 238.43 | 52.000 | 1.44 | 5.97 | Si VIII | N | 2p 4S3/2 | 4d 4P5/2,3/2,1/2 |
| 244.59 | 50.690 | 1.30 | 6.16 | Si X | B | 2p 2P3/2 | 3d 2D5/2 |
| 245.37 | 50.530 | 1.30 | 6.16 | Si X | B | 2p 2P1/2 | 3d 2D3/2 |
| 251.90 | 49.220 | 1.45 | 6.22 | Si XI | Be | 2p 1P1 | 3d 1D2 |
| 252.10 | 49.180 | 1.64 | 5.97 | Ar IX | Ne | 2p 1S0 | 3s 3P1,2 |
| 261.02 | 47.500 | 1.47 | 6.06 | S IX | O | 2p 3P | 3d 3D, 3P |
| 280.73 | 44.165 | 1.60 | 6.30 | Si XII | Li | 2p 2P3/2 | 3d 2D5/2 |
| 283.46 | 43.740 | 1.46 | 6.22 | Si XI | Be | 2s 1S0 | 3p 1P1 |
| E |  |
-log | log | ion | iso-el. | lower | upper |
| (eV) | (Å) | Qmax | Tmax | seq. | level | level | |
| 291.52 | 42.530 | 1.32 | 6.18 | S X | N | 2p 4S3/2 | 3d 4P5/2,3/2,1/2 |
| 298.97 | 41.470 | 1.31 | 5.97 | C V | He | 1s 1S0 | 2s 3S1 (f) |
| 303.07 | 40.910 | 1.75 | 6.29 | Si XII | Li | 2s 2S1/2 | 3p 2P3/2 |
| 307.88 | 40.270 | 1.27 | 5.98 | C V | He | 1s 1S0 | 2p 1P1 (r) |
| 315.48 | 39.300 | 1.37 | 6.28 | S XI | C | 2p 3P | 3d 3D, 1D, 3F |
| 336.00 | 36.900 | 1.58 | 6.19 | S X | N | 2p 4S3/2 | 4d 4P5/2,3/2,1/2 |
| 339.10 | 36.563 | 1.56 | 6.34 | S XII | B | 2p 2P3/2 | 3d 2D5/2 |
| 340.63 | 36.398 | 1.56 | 6.34 | S XII | B | 2p 2P1/2 | 3d 2D3/2 |
| 367.47 | 33.740 | 1.47 | 6.13 | C VI | H | 1s 2S1/2 | 2p 2P1/2
(Ly ) ) |
| 367.53 | 33.734 | 1.18 | 6.13 | C VI | H | 1s 2S1/2 | 2p 2P3/2
(Ly ) ) |
| 430.65 | 28.790 | 1.69 | 6.17 | N VI | He | 1s 1S0 | 2p 1P1 (r) |
| 500.36 | 24.779 | 1.68 | 6.32 | N VII | H | 1s 2S1/2 | 2p 2P3/2
(Ly ) ) |
| 560.98 | 22.101 | 0.86 | 6.32 | O VII | He | 1s 1S0 | 2s 3S1 (f) |
| 568.55 | 21.807 | 1.45 | 6.32 | O VII | He | 1s 1S0 | 2p 3P2,1 (i) |
| 573.95 | 21.602 | 0.71 | 6.33 | O VII | He | 1s 1S0 | 2p 1P1 (r) |
| 653.49 | 18.973 | 1.05 | 6.49 | O VIII | H | 1s 2S1/2 | 2p 2P1/2
(Ly ) ) |
| 653.68 | 18.967 | 0.77 | 6.48 | O VIII | H | 1s 2S1/2 | 2p 2P3/2
(Ly ) ) |
| 665.62 | 18.627 | 1.58 | 6.34 | O VII | He | 1s 1S0 | 3p 1P1 |
| 725.05 | 17.100 | 0.87 | 6.73 | Fe XVII | Ne | 2p 1S0 | 3s 3P2 |
| 726.97 | 17.055 | 0.79 | 6.73 | Fe XVII | Ne | 2p 1S0 | 3s 3P1 |
| 738.88 | 16.780 | 0.87 | 6.73 | Fe XVII | Ne | 2p 1S0 | 3s 1P1 |
| 771.14 | 16.078 | 1.37 | 6.84 | Fe XVIII | F | 2p 2P3/2 | 3s 4P5/2 |
| 774.61 | 16.006 | 1.55 | 6.50 | O VIII | H | 1s 2S1/2 | 3p 2P1/2,3/2 (Ly
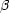 ) ) |
| 812.21 | 15.265 | 1.12 | 6.74 | Fe XVII | Ne | 2p 1S0 | 3d 3D1 |
| 825.79 | 15.014 | 0.58 | 6.74 | Fe XVII | Ne | 2p 1S0 | 3d 1P1 |
| 862.32 | 14.378 | 1.69 | 6.84 | Fe XVIII | F | 2p 2P3/2 | 3d 2D5/2 |
| 872.39 | 14.212 | 1.54 | 6.84 | Fe XVIII | F | 2p 2P3/2 | 3d 2S1/2 |
| 872.88 | 14.204 | 1.26 | 6.84 | Fe XVIII | F | 2p 2P3/2 | 3d 2D5/2 |
| 896.75 | 13.826 | 1.66 | 6.76 | Fe XVII | Ne | 2s 1S0 | 3p 1P1 |
| 904.99 | 13.700 | 1.61 | 6.59 | Ne IX | He | 1s 1S0 | 2s 3S1 (f) |
| 905.08 | 13.699 | 1.61 | 6.59 | Ne IX | He | 1s 1S0 | 2s 3S1 (f) |
| 916.98 | 13.521 | 1.35 | 6.91 | Fe XIX | O | 2p 3P2 | 3d 3D3 |
| 917.93 | 13.507 | 1.68 | 6.91 | Fe XIX | O | 2p 3P2 | 3d 3P2 |
| 922.02 | 13.447 | 1.44 | 6.59 | Ne IX | He | 1s 1S0 | 2p 1P1 (r) |
| 965.08 | 12.847 | 1.51 | 6.98 | Fe XX | N | 2p 4S3/2 | 3d 4P5/2 |
| 966.59 | 12.827 | 1.44 | 6.98 | Fe XX | N | 2p 4S3/2 | 3d 4P3/2 |
| 1009.2 | 12.286 | 1.12 | 7.04 | Fe XXI | C | 2p 3P0 | 3d 3D1 |
| 1011.0 | 12.264 | 1.46 | 6.73 | Fe XVII | Ne | 2p 1S0 | 4d 3D1 |
| 1021.5 | 12.137 | 1.77 | 6.77 | Ne X | H | 1s 2S1/2 | 2p 2P1/2
(Ly ) ) |
| 1022.0 | 12.132 | 1.49 | 6.76 | Ne X | H | 1s 2S1/2 | 2p 2P3/2
(Ly ) ) |
| 1022.6 | 12.124 | 1.39 | 6.73 | Fe XVII | Ne | 2p 1S0 | 4d 1P1 |
| 1053.4 | 11.770 | 1.38 | 7.10 | Fe XXII | B | 2p 2P1/2 | 3d 2D3/2 |
| 1056.0 | 11.741 | 1.51 | 7.18 | Fe XXIII | Be | 2p 1P1 | 3d 1D2 |
| 1102.0 | 11.251 | 1.73 | 6.74 | Fe XVII | Ne | 2p 1S0 | 5d 3D1 |
| 1352.1 | 9.170 | 1.66 | 6.81 | Mg XI | He | 1s 1S0 | 2p 1P1 (r) |
| 1472.7 | 8.419 | 1.76 | 7.00 | Mg XII | H | 1s 2S1/2 | 2p 2P3/2
(Ly ) ) |
| 1864.9 | 6.648 | 1.59 | 7.01 | Si XIII | He | 1s 1S0 | 2p 1P1 (r) |
| 2005.9 | 6.181 | 1.72 | 7.21 | Si XIV | H | 1s 2S1/2 | 2p 2P3/2
(Ly ) ) |
| 6698.6 | 1.851 | 1.43 | 7.84 | Fe XXV | He | 1s 1S0 | 2p 1P1 (r) |
| 6973.1 | 1.778 | 1.66 | 8.17 | Fe XXVI | H | 1s 2S1/2 | 2p 2P3/2
(Ly ) ) |